Manufacturing





Sundyota Numandis
Located in Ahmedabad, India’s largest pharmaceutical hub, with a total built-up area of 80,000 sq. ft.
- We have dedicated Research, Manufacturing, and Utility blocks, all compliant with current FSSAI regulations and cGMP standards for food product manufacturing.
- Specialized in the formulation development and manufacturing of Nutraceuticals and Probiotics (non-spore). We collaborate with European scientists to create cutting-edge formulations.
- Designed to be Vaastu compliant, our facility fosters positive energy flow, contributing to prosperity and well-being for all.
Our Products
We put our Heart and Soul into pioneering products that truly add value to the therapies that they cater to. Our products are Patented, In-licensed with exclusivity, Globally acclaimed with Clinically proven efficacy and Safety.
Our Departments
- Inventory Management
- Manufacturing & Packaging Engineering
- Human Resources
- Environment, Health & Safety
- Information Technology
- Purchase & Planning
- Project Management
- Formulation & Development
- Analytical Development
- Packaging Development
- Quality Control
- Quality Assurance
- Regulatory Affairs
Manufacturing
The manufacturing facility spans a total area of 23,095 sq. ft, featuring clean room modular partitioning with epoxy flooring. It is equipped with temperature and humidity control, maintaining conditions as low as 20% RH, and includes designated Class D manufacturing areas.
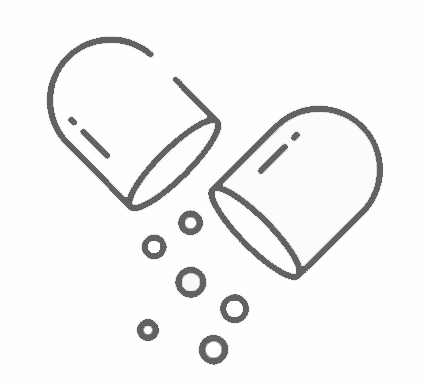
Up to 1 billion per year.
Up to 1.8 million kg of blends per year.
150 million sachets and sticks per year.

Up to 425 million per year.

Up to 10 million suspension and emulsion bottles per year.
Inventory Management
The warehouse has a storage capacity of 1,300 pallets, featuring clean room modular partitioning and vacuum de-watered flooring.
It includes temperature and humidity-controlled areas, with separate sections for raw materials, primary packaging materials, empty capsules, secondary packaging materials, and finished goods.
A dedicated walk-in refrigerated chamber (2–8°C) is available for the storage of temperature-sensitive materials.
The facility ensures streamlined operations with separate paths for material and personnel movement and distinct elevators for the transport of raw materials and finished goods.



Formulation & Development
DEVELOPMENT CAPABILITIES
DEVELOPMENT UNDER CARE
Quality
SOP-based processes are adhered to by every department within our organization.
Quality attributes are thoroughly verified at multiple stages, including raw materials and finished products. Analytical testing methods are verified and validated, with quality checks conducted alongside stability studies at various stations.
Climate-controlled chambers are used for stability studies under long-term and accelerated conditions, equipped with an auto-alarm facility.
We follow a set of standards, including HACCP, GHP, and GMP, to manage food safety in compliance with FSSC 22000 v6, ensuring a high level of assurance within the global food supply chain.
Quality xCertification
Located in Ahmedabad, India’s largest pharmaceutical hub, our facility spans a total built-up area of 80,000 sq. ft.
-FSSAI Schedule IV: An ‘exemplar’ facility compliant with FSSAI requirements for food manufacturers.
-Food cGMP: Ensures consistency in manufacturing processes to maintain product quality.
-FSSC 22000 Ver. 6: A globally recognized certification for food safety management systems.
-US FDA Registered Facility: Compliant with US FDA standards for manufacturing.